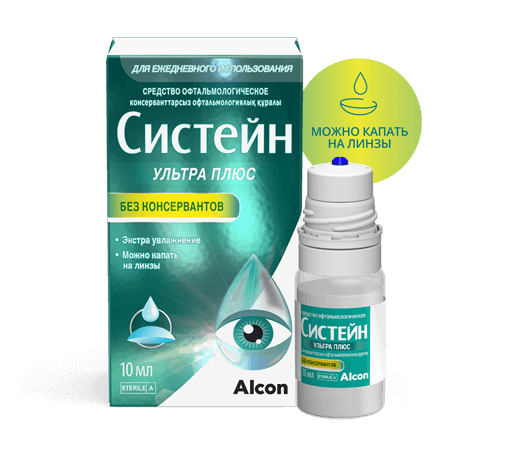
Систейн Ультра Плюс
без консервантов
без консервантов
Состав: Гиалуроновая кислота 0,15%, Гидроксипропилгуар 0,175%, Полиэтиленгликоль 400, Пропиленгликоль, Сорбитол, Аминометилпропанол, Борная кислота, Натрия борат, Калия хлорид, Натрия хлорид
Производитель: Alcon Inc, США
Срок хранения вскрытого флакона: 3 месяца
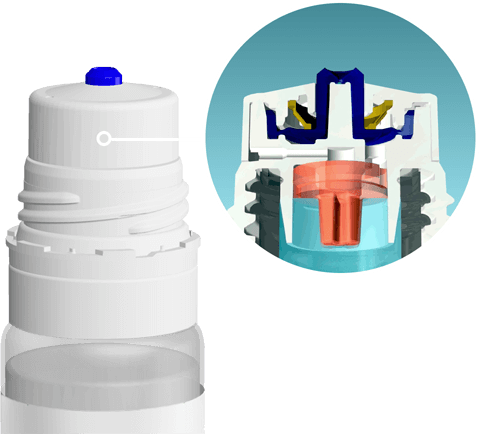
Объем флакона: 10 мл
Можно ли капать на контактные линзы: да
Срок хранения вскрытого флакона: 3 месяца
Объем флакона: 10 мл
Можно ли капать на контактные линзы: да
Ключевые отличия технологии Систейн
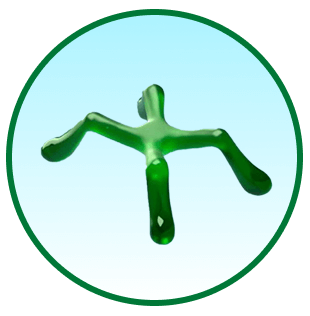
Уникальная
полимерная сеть1
полимерная сеть1
Мукомиметические свойства3,7

pH-адаптивность4,7
Усиленное действие гиалуроновой кислоты2
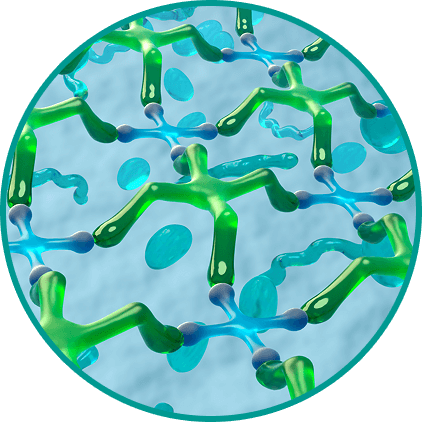
Механизм действия Систейн Ультра Плюс
-
Усиленное действие за счет двух полимерных
увлажнителей —
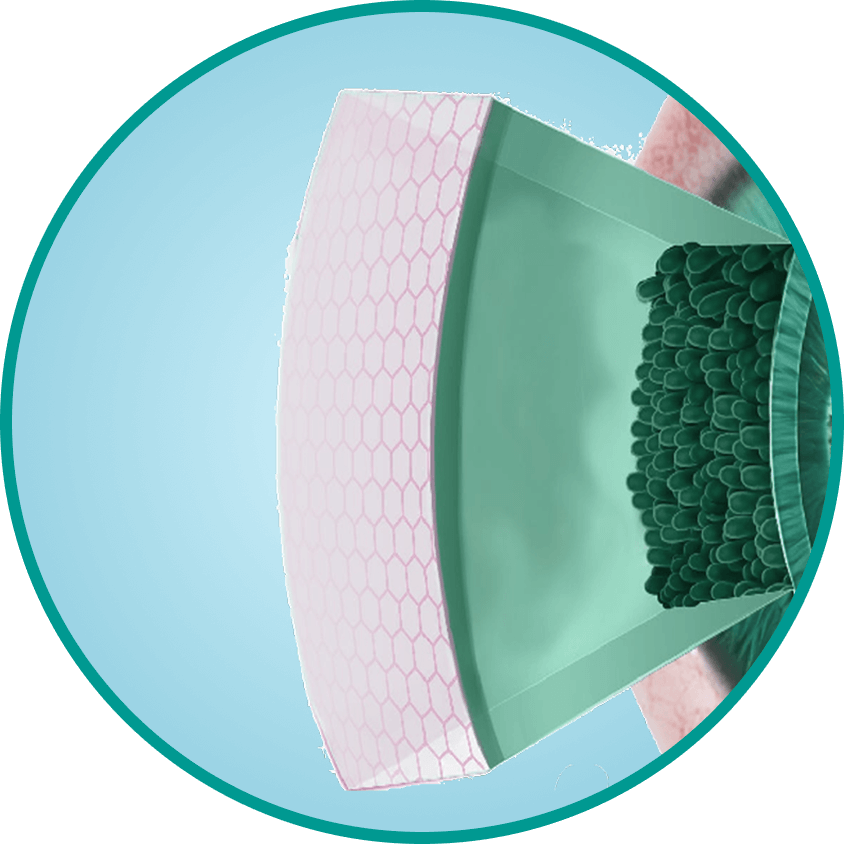
ГП-Гуара и гиалуроновой кислоты2 - Формирует на поверхности глаза гелеобразный защитный матрикс, повторяя свойства гликокаликса роговицы3
- Обеспечивает эффект «умной доставки» активных компонентов, продлевая увлажнение и защиту5

Показания к применению
Синдром сухого глаза
Синдром сухого глаза средней/тяжелой степени с дефицитом водно-муцинового компонента слезы10
Подготовка к офтальмологическим операциям
Улучшает состояние глазной поверхности и повышает точность диагностики12
Постоянные симптомы дискомфорта и сухости глаз
Обеспечивает защиту глазной поверхности. Улучшает качество зрения. Повышает стабильность слезной пленки
В постоперационном периоде
Способствует восстановлению глазной поверхности и устранению симптомов дискомфорта11,12
Использование контактных линз
Обеспечивает увлажнение линз и удержание влаги на их поверхности. Можно капать на линзы в процессе ношения. Совместим со всеми видами мягких контактных линз
Вторичный синдром сухого глаза
Вследствие конъюнктивитов различной этиологии, глаукомы, сахарного диабета, аллергии, и других заболеваний
Кому подходит Систейн Ультра Плюс?

Постоянные симптомы
- Пациенты с ССГ, страдающие от постоянных симптомов сухости и дискомфорта в глазах
- Пациенты, у которых симптомы усиливаются к вечеру, особенно после длительной зрительной нагрузки
- Пациенты с жалобами на недостаточную эффективность и длительность действия увлажняющих капель

Вторичный ССГ
- Вторичный ССГ, связанный с повреждением глазной поверхности различной этиологии (аллергия, сахарный диабет)
- Ятрогенный ССГ (глаукома, состояние после интравитреальных инъекций и глазной хирургии)

До и после операций на глазах
- Пациенты, планирующие операции на глазах (хирургия катаракты, глаукомы, рефракционные операции)
- Пациенты после операций на глазах, нуждающиеся в облегчении симптомов глазного дискомфорта
Клинически доказанная эффективность

В 2 раза эффективнее
удерживает влагу2
удерживает влагу2
по сравнению с гиалуроновой
кислотой
кислотой

Лучшее заживление
и реэпителизация роговицы8,12
и реэпителизация роговицы8,12
по сравнению с каплями на основе гиалуроновой кислоты

Выше стабильность
слезной пленки14
слезной пленки14
по сравнению с каплями на основе гиалуроновой кислоты

Более быстрое восстановление глазной поверхности12
после операций на глазах
1. Патенты № US8685945B2 от 01.04.2014, US10828320B2 от 10.11.2020, US005397567A от 14.03.1995
2. Rangarajan R, Kraybill B, Ogundele A, Ketelson HA. Effects of a hyaluronic acid/hydroxypropyl guar artificial tear solution on protection, recovery, and lubricity in models of corneal epithelium. J Ocul Pharmacol Ther. 2015;31(8): 491–497. doi:10.1089/jop.2014.0164
3. Moon SW, Hwang JH, Chung SH, Nam KH. The impact of artificial tears containing hydroxypropyl guar on mucous layer. Cornea. 2010;29(12): 1430. doi:10.1097/ICO.0b013e3181ca636b
4. Srinivasan, Sruthi and Venkiteshwar Manoj. «A Decade of Effective Dry Eye Disease Management with Systane Ultra (Polyethylene Glycol/Propylene Glycol with Hydroxypropyl Guar) Lubricant Eye Drops. Clinical Ophthalmology (Auckland, N.Z.) 15 (2021): 2421 — 2435.
5. Springs C. Novel ocular lubricant containing an intelligent delivery system: details of its mechanism of action. Dev Ophthalmol. 2010;45:139–147. doi: 10.1159/000315027. Epub 2010 May 18. PMID: 20502034.
6. Petricek I, Berta A, Higazy MT, Németh J, Prost ME. Hydroxypropyl-guar gellable lubricant eye drops for dry eye treatment. Expert Opin Pharmacother. 2008 Jun;9(8): 1431–6. doi: 10.1517/14656566.9.8.1431. PMID: 18473717.
7. Ng A, Keech A, Jones L. Tear osmolarity changes after use of hydroxypropyl-guar-based lubricating eye drops. Clin Ophthalmol. 2018 Apr 10;12:695–700. doi: 10.2147/OPTH.S150587. PMID: 29692601; PMCID: PMC5903481.
8. Carlson E, Kao WWY, Ogundele A. Impact of hyaluronic acid-containing artificial tear products on reepithelialization in an in vivo corneal wound model. J Ocul Pharmacol Ther. 2018;34(4): 360–364. doi:10.1089/jop.2017.0080
9. Patterson B, Crary M, Shannon P. Microbial Ingress Risk Evaluation of Novelia Package Containing Systane Ultra. Invest Ophthalmol Vis Sci. 2021; 62:1303. doi: 10.1016/j.clae.2022.101660
10. Yokoi N, Yamada H, Mizukusa Y, et al. Rheology of Tear Film Lipid Layer Spread in Normal and Aqueous Tear-Deficient Dry Eyes. Invest Ophthalmol Vis Sci. 2008;49(12): 5319–5324. doi:10.1167/iovs.07–1407.
11. Rolando M, Autori S, Badino F, Barabino S. Protecting the ocular surface and improving the quality of life of dry eye patients: a study of the efficacy of an hp-guar containing ocular lubricant in a population of dry eye patients. J Ocul Pharmacol Ther. 2009;25(3): 271–278. doi:1089/jop.2008.0026
12. Sun, CC., Chan, YH., Huang, PW. et al. Evaluation of two artificial tears containing Hyaluronic Acid for post cataract surgery dry eye disease: A randomized controlled trial. Ophthalmol Ther 13, 2615–2627 (2024). https://doi.org/10.1007/s40123-024-01015-9
13. Starr CE, Gupta PK, Farid M, Beckman KA, Chan CC, Yeu E, Gomes JAP, Ayers BD, Berdahl JP, Holland EJ, Kim T, Mah FS, ASCRS Cornea Clinical Committee. An algorithm for the preoperative diagnosis and treatment of ocular surface disorders. J Cataract Refract Surg 2019;45:669–84.
14. Jia T, Stapleton F, Iqbal F, Showyin J, Roy D, Roy M, Tan J. Comparison of eye drop retention time using fluorophotometry in three commercially available lubricant eye drops. Optom Vis Sci. 2024 Aug 28. doi: 10.1097/OPX.0000000000002172. Epub ahead of print. PMID: 39190794.
2. Rangarajan R, Kraybill B, Ogundele A, Ketelson HA. Effects of a hyaluronic acid/hydroxypropyl guar artificial tear solution on protection, recovery, and lubricity in models of corneal epithelium. J Ocul Pharmacol Ther. 2015;31(8): 491–497. doi:10.1089/jop.2014.0164
3. Moon SW, Hwang JH, Chung SH, Nam KH. The impact of artificial tears containing hydroxypropyl guar on mucous layer. Cornea. 2010;29(12): 1430. doi:10.1097/ICO.0b013e3181ca636b
4. Srinivasan, Sruthi and Venkiteshwar Manoj. «A Decade of Effective Dry Eye Disease Management with Systane Ultra (Polyethylene Glycol/Propylene Glycol with Hydroxypropyl Guar) Lubricant Eye Drops. Clinical Ophthalmology (Auckland, N.Z.) 15 (2021): 2421 — 2435.
5. Springs C. Novel ocular lubricant containing an intelligent delivery system: details of its mechanism of action. Dev Ophthalmol. 2010;45:139–147. doi: 10.1159/000315027. Epub 2010 May 18. PMID: 20502034.
6. Petricek I, Berta A, Higazy MT, Németh J, Prost ME. Hydroxypropyl-guar gellable lubricant eye drops for dry eye treatment. Expert Opin Pharmacother. 2008 Jun;9(8): 1431–6. doi: 10.1517/14656566.9.8.1431. PMID: 18473717.
7. Ng A, Keech A, Jones L. Tear osmolarity changes after use of hydroxypropyl-guar-based lubricating eye drops. Clin Ophthalmol. 2018 Apr 10;12:695–700. doi: 10.2147/OPTH.S150587. PMID: 29692601; PMCID: PMC5903481.
8. Carlson E, Kao WWY, Ogundele A. Impact of hyaluronic acid-containing artificial tear products on reepithelialization in an in vivo corneal wound model. J Ocul Pharmacol Ther. 2018;34(4): 360–364. doi:10.1089/jop.2017.0080
9. Patterson B, Crary M, Shannon P. Microbial Ingress Risk Evaluation of Novelia Package Containing Systane Ultra. Invest Ophthalmol Vis Sci. 2021; 62:1303. doi: 10.1016/j.clae.2022.101660
10. Yokoi N, Yamada H, Mizukusa Y, et al. Rheology of Tear Film Lipid Layer Spread in Normal and Aqueous Tear-Deficient Dry Eyes. Invest Ophthalmol Vis Sci. 2008;49(12): 5319–5324. doi:10.1167/iovs.07–1407.
11. Rolando M, Autori S, Badino F, Barabino S. Protecting the ocular surface and improving the quality of life of dry eye patients: a study of the efficacy of an hp-guar containing ocular lubricant in a population of dry eye patients. J Ocul Pharmacol Ther. 2009;25(3): 271–278. doi:1089/jop.2008.0026
12. Sun, CC., Chan, YH., Huang, PW. et al. Evaluation of two artificial tears containing Hyaluronic Acid for post cataract surgery dry eye disease: A randomized controlled trial. Ophthalmol Ther 13, 2615–2627 (2024). https://doi.org/10.1007/s40123-024-01015-9
13. Starr CE, Gupta PK, Farid M, Beckman KA, Chan CC, Yeu E, Gomes JAP, Ayers BD, Berdahl JP, Holland EJ, Kim T, Mah FS, ASCRS Cornea Clinical Committee. An algorithm for the preoperative diagnosis and treatment of ocular surface disorders. J Cataract Refract Surg 2019;45:669–84.
14. Jia T, Stapleton F, Iqbal F, Showyin J, Roy D, Roy M, Tan J. Comparison of eye drop retention time using fluorophotometry in three commercially available lubricant eye drops. Optom Vis Sci. 2024 Aug 28. doi: 10.1097/OPX.0000000000002172. Epub ahead of print. PMID: 39190794.
RU-SYH-2500003
Раздел только
для специалистов
Внимание!
Данный раздел сайта содержит информацию, предназначенную только для медицинских и фармацевтических работников.
Вы являетесь медицинским или фармацевтическим работником?
Соединение с интернетом отсутствует